Results for 'FDA anticancer approvals'



Evaluation of Trials Comparing Single-Enantiomer Drugs to Their Racemic Precursors: A Systematic Review
May 6th • 18 mins read

Updated estimates of eligibility for and response to genome-targeted oncology drugs among US cancer patients, 2006-2020
Apr 20th • 7 mins read
Comparative study on anticancer drug access times between FDA, EMA and the French temporary authorisation for use program over 13 years
Apr 7th • 12 mins read

Assessment of Coverage in England of Cancer Drugs Qualifying for US Food and Drug Administration Accelerated Approval
Feb 22nd • 10 mins read

Assessment of Food and Drug Administration- and European Medicines Agency-Approved Systemic Oncology Therapies and Clinically Meaningful Improvements in Quality of Life: A Systematic Review
Feb 11th • 4 mins read
Accelerated drug approvals in oncology: Pros and cons
Sep 14th • 4 mins read
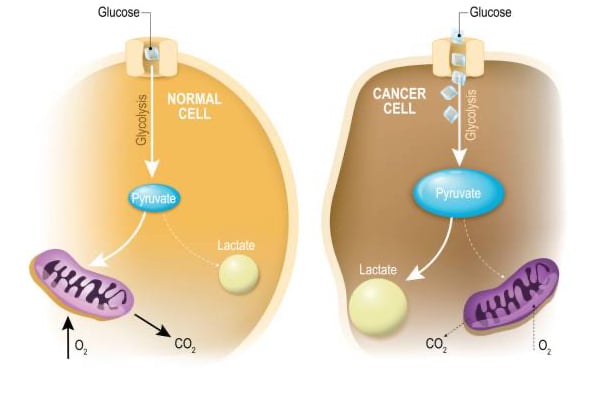
“Oncometabolism: The switchboard of cancer: An editorial”
Feb 1st • 1 min read
Loose Regulatory Standards Portend a New Era of Imprecision Oncology
Dec 1st • 4 mins read
A narrative review of biosimilars: a continued journey from the scientific evidence to practice implementation
Aug 3rd • 10 mins read

Liquid biopsy in oncology: a consensus statement of the Spanish Society of Pathology and the Spanish Society of Medical Oncology
Sep 26th • 17 mins read

Application of the ESMO-Magnitude of Clinical Benefit Scale (V.1.1) to the field of early breast cancer therapies
Sep 6th • 20 mins read
Clinical benefit of immune checkpoint inhibitors approved by US Food and Drug Administration
Aug 31st • 16 mins read

Past, Current, and Future Cancer Clinical Research Collaborations: The Case of the European Organisation for Research and Treatment of Cancer
Aug 16th • 8 mins read

Clinical benefit and cost of breakthrough cancer drugs approved by the US Food and Drug Administration
Jul 22nd • 12 mins read
Comparison of Access to Novel Drugs for Lymphoma and Chronic Lymphocytic Leukemia Between India and the United States
Jul 21st • 12 mins read

Biosimilars in oncology: key role of nurses in patient education
Jun 15th • 10 mins read

Real-World Evidence: Bridging Gaps in Evidence to Guide Payer Decisions
Jun 18th • 6 mins read

The regulatory landscape of precision oncology laboratory medicine in the United States - Perspective on the past 5 years and considerations for future regulation
May 22nd • 8 mins read
